First new antibiotic in 50 years enters final tests to fight deadly superbug

Roche hopes the drug will receive approval by the end of the decade
Don't Miss
Most Read
The first new antibiotic in 50 years to tackle a common superbug is entering its final phase of human testing.
Swiss pharmaceutical giant Roche announced on Monday that zosurabalpin will now progress to phase-three trials.
The drug targets Acinetobacter baumannii, a pathogen described as a "priority" by the World Health Organisation and an "urgent threat" by the US Center for Disease Control and Prevention.
This drug-resistant bacteria primarily affects hospital patients, causing serious infections including pneumonia and sepsis.

The drug will undergo late-stage human trials
|GETTY
Between 40 and 60 per cent of infected patients die as a result of the bug, with many being immunocompromised due to conditions such as cancer.
The development has been hailed as an "exciting" breakthrough in the fight against antibiotic resistance, which world leaders recently declared "one of the most urgent global health threats".
Zosurabalpin works differently from all existing antibiotics by targeting the "machine" that prevents the bacteria's outer membrane from forming properly.
One of the main challenges in treating Acinetobacter baumannii is its double-walled membrane that protects it from attack.
The drug, developed in collaboration with Harvard University researchers, could potentially lay the foundations for future antibiotics.
Michael Lobritz, global head of infectious diseases at Roche, said: "Our goal is to contribute new innovations to overcome antimicrobial resistance, one of the biggest infectious disease challenges to public health."
Larry Tsai from Genentech, a Roche unit, added that the "innovative biology involved in this research could potentially reveal new insights into the structure of bacterial membranes, possibly leading to the discovery of new antibiotics in the future".
The phase-three trial, expected to begin later this year or in early 2026, will involve approximately 400 patients with carbapenem-resistant Acinetobacter Baumannii (CRAB) infection. Participants will either receive zosurabalpin or the current standard of care.
LATEST DEVELOPMENTS:
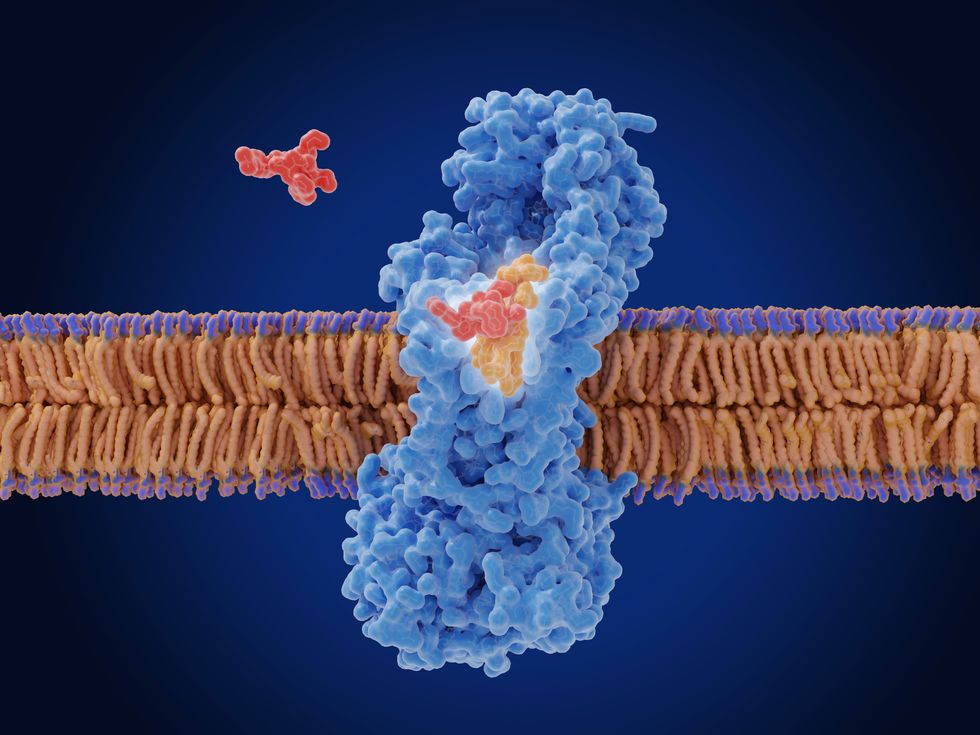
An illustration showing how zosurabalpin (in red) stops the outer layer of the bacterial cell wall from forming properly
|GETTY
Roche hopes the drug will receive approval by the end of the decade.
Dr Alistair Farley, scientific lead at the Ineos Oxford Institute, welcomed zosurabalpin as an "exciting development" for the field, noting "there is an urgent unmet clinical need to develop new antibiotics against priority pathogens such as CRAB, where antimicrobial resistance is a major concern".
Earlier studies published in Nature demonstrated the drug worked "extremely well" in test tubes and mice.
Professor Laura Piddock, scientific director of the Global Antibiotic Research and Development Partnership, previously stated it provided "definite hope" for other hard-to-treat infections.
The UN has warned that without action, drug-resistant diseases could cause 10 million deaths annually by 2050.