Alzheimer’s breakthrough: Treatment enters ‘new era’ as drug provides ‘turning point’

Alzheimer's patients could receive a new boost as a drug provides a 'turning point'
|PA

Around 900,000 people in the United Kingdom suffer from dementia
Don't Miss
Most Read
Latest
A new drug could provide a “turning point” for treating Alzheimer’s disease, experts have claimed.
Donanemab was found to slow “clinical decline” by as much as 35 per cent in a potential boost to hundreds of thousands of Britons battling with the disease.
The drug could help people struggling with Alzheimer’s perform day-to-day tasks, including shopping, housekeeping, managing finances and taking medication.
Charity Alzheimer’s Research UK responded to the development by saying “we’re entering a new era where Alzheimer’s disease could become treatable”.
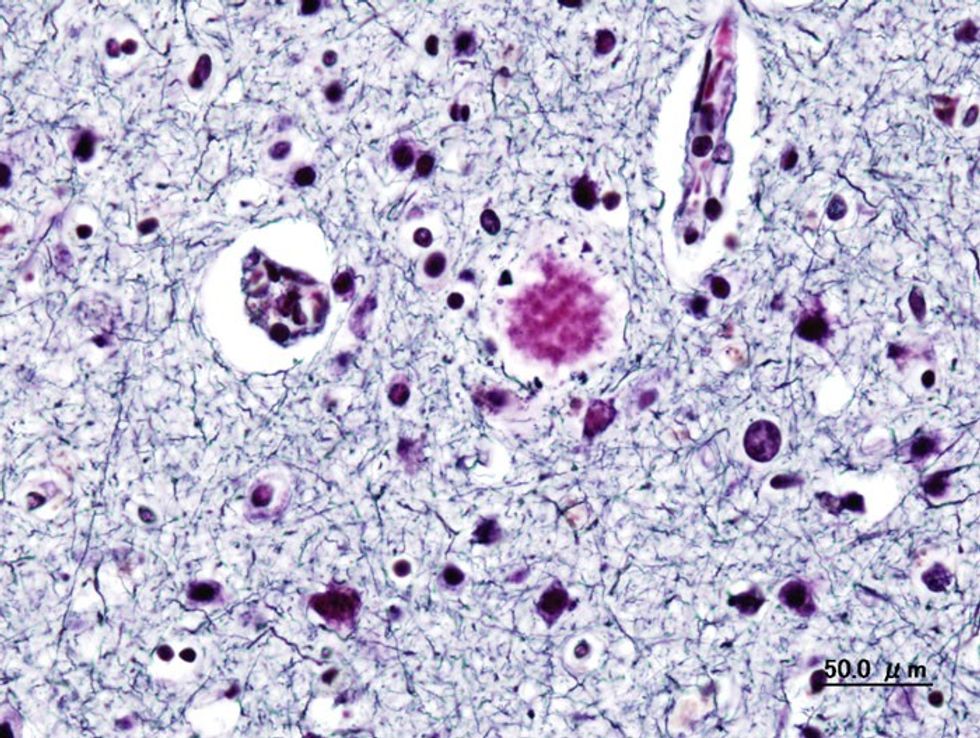
Histopathogic image of senile plaques seen in the cerebral cortex in a patient with presenile onset of Alzheimer disease
|Wikimedia Commons Licences
Alzheimer’s Society also hopes the slowdown could result in the disease being compared to asthma or diabetes.
Donanemab works by removing a protein called amyloid.
The protein builds up in the brain and results in the deterioration of sufferers.
Scientists recently published the final results of a trial assessing the safety and efficacy of donanemab, called TRAILBLAZER ALZ-2.
Almost 1,800 people with early-stage Alzheimer’s were included in the study.
Half received a monthly infusion of donanemab and the other half were given a placebo over an 18-month period.
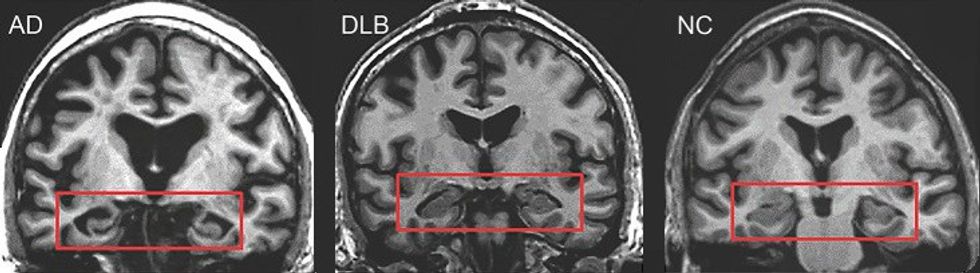
MRI of brain showing hippocampus atrophy (red rectangles), more prominent in Alzheimer's disease
|Wikimedia Commons Licences
The Journal of the American Medication Association presented the study to the Alzheimer’s Association International Conference in Amsterdam.
It concluded donanemab was able to slow clinical decline by 35.1 per cent in people whose brain scans showed low or medium levels of a protein called tau.
Alzheimer’s progression was slowed by 22.3 per cent when the results included people who had different levels of tau.
However, researchers found some participants in the study suffered from severe side effects.
Brain swelling was among the potential side effects and three people in the donanemab group were registering as having “treatment-related” deaths.
A participant from the placebo group was also recorded as having a “treatment-related” death.
 The breakthrough drug could help alleviate Alzheimer's disease | Pexels
The breakthrough drug could help alleviate Alzheimer's disease | PexelsDr Mark Mintun, group vice president of neuroscience research and development at Lilly and president of Avid Radiopharmaceuticals, said: “People living with early, symptomatic Alzheimer’s disease are still working, enjoying trips, sharing quality time with family – they want to feel like themselves, for longer. The results of this study reinforce the importance of diagnosing and treating disease sooner than we do today.”
Dr Richard Oakley, associate director of research and innovation at Alzheimer’s Society, claimed: “This is truly a turning point in the fight against Alzheimer’s and science is proving that it is possible to slow down the disease.
“Treatments like donanemab are the first steps towards a future where Alzheimer’s disease could be considered a long-term condition alongside diabetes or asthma – people may have to live with it, but they could have treatments that allow them to effectively manage their symptoms and continue to live fulfilled lives.
“Today’s full results support what we heard about donanemab back in May, that the drug is able to slow down the progression of Alzheimer’s disease by more than 20 per cent.
“This study adds to the growing evidence that treating people as early as possible may be more beneficial, with the effects of donanemab greater in people who were at an earlier stage of the disease.
“Diagnosis will be key to the access of any new treatments. We can’t have a situation where treatments are approved for use in the UK but people aren’t diagnosed early or accurately enough to be eligible.
 The number of people suffering with Alzheimer’s in the United Kingdom is estimated to total 850,000 | PA
The number of people suffering with Alzheimer’s in the United Kingdom is estimated to total 850,000 | PA“We need early, and accurate, diagnoses available for everyone and the NHS ready to roll out treatments such as donanemab and lecanemab if and when they are approved in the UK.”
Dr Susan Kohlhaas, executive director of research and partnerships at Alzheimer’s Research UK, added: “Today’s announcement marks another milestone.
“Thanks to decades of research, the outlook for dementia and its impact on people and society is finally changing, and we’re entering a new era where Alzheimer’s disease could become treatable.
“As a potential first-generation treatment, donanemab’s effects are modest.
“But these results provide further confirmation that removing amyloid from the brain can change the course of Alzheimer’s, and may help people affected by this devastating disease if they’re treated at the right time.
“Set against this, it’s clear that donanemab does come with side effects, which for some can be very serious.
“Regulators will need to balance these benefits and risks before it is given a license for use.
The breakthrough comes shortly after another drug, known as lecanemab, was found to reduce monthly memory decline among patients with early-stage Alzheimer’s disease.
American regulators approved the drug earlier this month and it had been suggested the UK should look into whether it could be used to help Britons.










