New guidance issued for weight loss jabs as regulator urges vigilance over pancreatitis symptoms

Patients and clinicians are being urged to remain vigilant for warning signs
Don't Miss
Most Read
Britain's medicines regulator has revised its guidance on popular weight-loss and diabetes injections following a surge in reports of acute pancreatitis linked to the treatments.
The Medicines and Healthcare products Regulatory Agency announced on Thursday that its yellow card monitoring scheme has received 1,143 reports of pancreatitis among patients using semaglutide or tirzepatide, the active ingredients in widely prescribed medications including Wegovy, Ozempic and Mounjaro.
Seventeen deaths have been recorded among those affected. The agency stressed that while the condition remains rare, patients and clinicians must remain vigilant for warning signs.
Patient information leaflets for these GLP-1 medications classify pancreatitis as an "uncommon" side effect, potentially affecting up to one in every hundred users.
Pancreatitis could potentially affect up to one in every hundred users
|GETTY
Acute pancreatitis occurs when the pancreas, a digestive gland situated behind the stomach, becomes suddenly inflamed.
Those affected typically experience intense abdominal pain, nausea and fever, with many requiring hospital admission.
Research indicates that approximately 1.6 million adults across England, Wales and Scotland were prescribed GLP-1 medications between early 2024 and early 2025.
The overwhelming majority of pancreatitis reports emerged this year, with 973 cases logged in 2025 alone. Of these, tirzepatide accounted for 807 reports while semaglutide was linked to 166 cases.
Additional reports have been filed concerning other GLP-1 treatments, with 146 cases involving liraglutide and 61 relating to dulaglutide.
The MHRA's yellow card scheme serves as the nation's primary system for tracking adverse reactions to medicines and medical devices.
Dr Alison Cave, the MHRA's chief safety officer, emphasised that GLP-1 medications remain safe and effective for the overwhelming majority of patients, delivering substantial health benefits.
She stated: "The risk of developing these severe side effects is very small, but it is important that patients and healthcare professionals are aware and alert to the associated symptoms."
The regulator has urged anyone taking these medications to seek medical advice promptly if they experience concerning symptoms.
LATEST DEVELOPMENTS
Dr Dave added: "If you, or someone you care for, is taking GLP-1s and you notice symptoms such as severe, persistent stomach pain that may radiate to the back and may be accompanied by nausea and vomiting, then we advise you speak to a healthcare professional and report it via our yellow card scheme."
Novo Nordisk, the manufacturer of Wegovy and Ozempic, responded by affirming its commitment to patient welfare.
"Patient safety is of the utmost importance to Novo Nordisk. We recommend that patients take these medications only for their approved indications and under the strict supervision of a healthcare professional, who can also advise on potential side effects," a company spokesperson said.
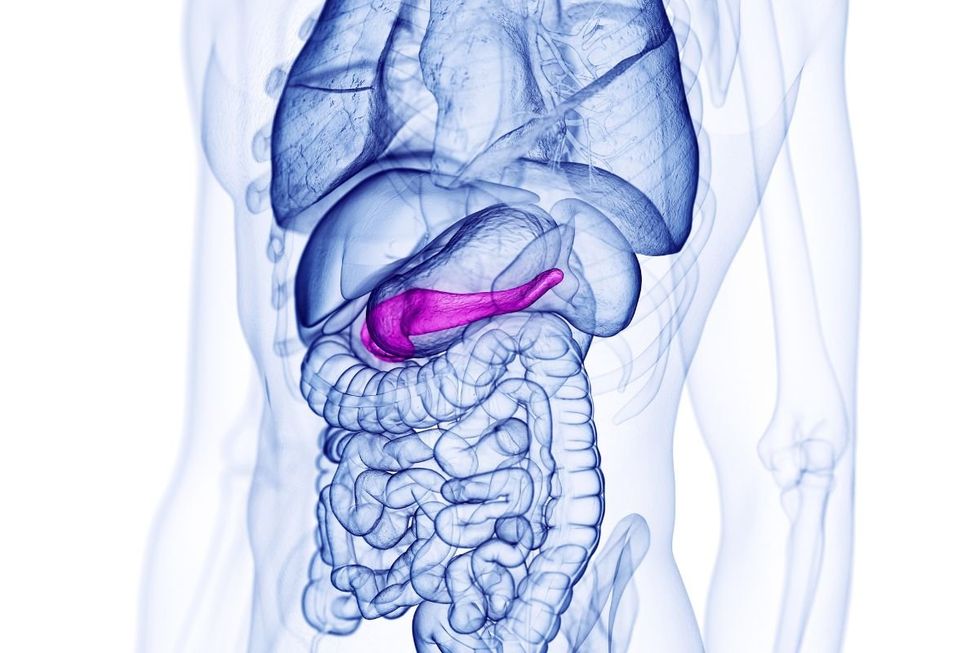
Acute pancreatitis occurs when the pancreas becomes suddenly inflamed
|GETTY
Eli Lilly, which produces Mounjaro, similarly stressed that patient safety remains its foremost concern and encouraged users to consult healthcare professionals about any side effects.
Meanwhile, the MHRA has enrolled GLP-1 patients in the Yellow Card Biobank study, a joint initiative with Genomics England.
The research aims to determine whether genetic factors influence an individual's susceptibility to pancreatic inflammation, potentially enabling more personalised prescribing decisions in future.
Our Standards: The GB News Editorial Charter










