Injection restores hearing within a month 'in huge step forward' for patients with genetic deafness

Children aged five to eight showed the most dramatic responses
Don't Miss
Most Read
Latest
A revolutionary gene therapy has restored hearing in ten patients suffering from congenital deafness, marking a significant breakthrough in treating genetic hearing loss.
The groundbreaking treatment, detailed in Nature Medicine, targeted individuals aged between one and 24 years who had severe hearing impairment caused by mutations in the OTOF gene.
The therapy involved a single injection of a synthetic virus carrying a functional version of the gene directly into the inner ear.
Results emerged remarkably quickly, with most patients experiencing improved hearing within just one month of treatment.

The therapy involved a single injection of a synthetic virus
|GETTY
The research involved patients at five Chinese hospitals who all shared a common genetic defect.
Mutations in their OTOF gene prevented production of otoferlin, a protein essential for transmitting sound signals from the ear to the brain.
The treatment utilised an adeno-associated virus (AAV) as a delivery vehicle. Doctors injected this modified virus through the round window, a membrane at the cochlea's base, allowing the therapeutic gene to reach the inner ear.
The study included participants ranging from toddlers to young adults, providing crucial data about the therapy's effectiveness across different age groups.
The treatment delivered remarkable outcomes, with participants' hearing thresholds improving from an average of 106 decibels to 52 decibels after six months. Children aged five to eight showed the most dramatic responses.
One seven-year-old girl experienced near-complete hearing restoration. "She was able to hold daily conversations with her mother four months afterwards," the study revealed.
The therapy proved effective across all age groups, including teenagers and adults receiving treatment for the first time.
"Hearing was greatly improved in many of the participants, which can have a profound effect on their life quality," said Dr Maoli Duan, a consultant at Karolinska Institutet and corresponding author.
The treatment demonstrated an excellent safety profile, with no serious adverse reactions reported during the six to 12-month follow-up period.
LATEST DEVELOPMENTS
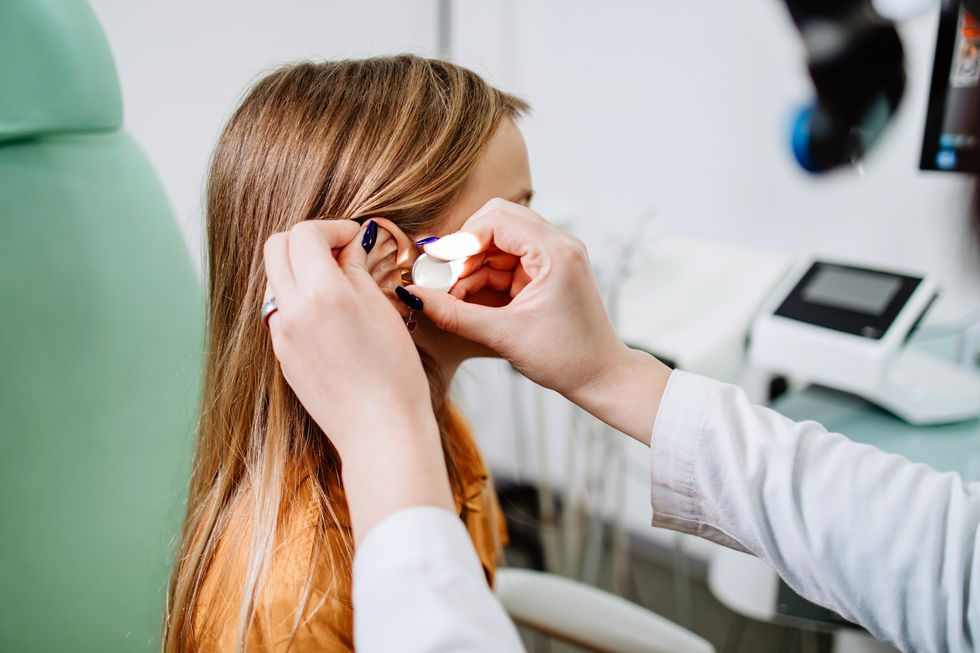
The injection represents a huge step forward in the genetic treatment of deafness
|GETTY
The most common side effect was a temporary reduction in neutrophil white blood cells.
"This is a huge step forward in the genetic treatment of deafness, one that can be life-changing for children and adults," Dr Duan stated. He emphasised that this represents just the beginning of genetic deafness treatments.
Researchers are already expanding their work to target other deafness-causing genes, including GJB2 and TMC1.
"We are confident that patients with different kinds of genetic deafness will one day be able to receive treatment," Dr Duan added.