Inhalers urgently recalled after major labelling error discovered on several batches

The error affects only printed information on cartons, not the inhaler contents themselves
Don't Miss
Most Read
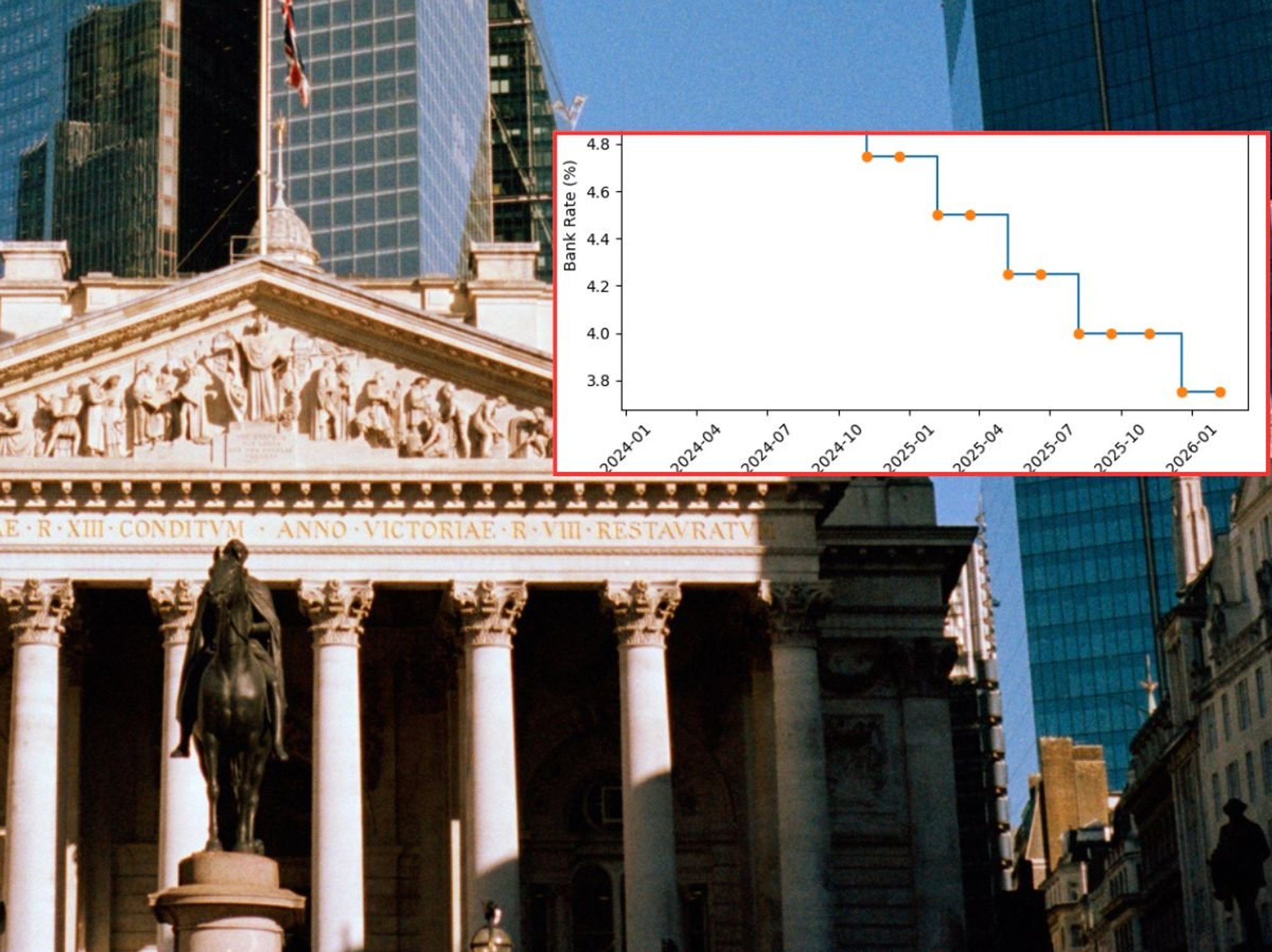
Britain's medicines regulator has ordered the withdrawal of four production runs of Flutiform asthma inhalers after discovering packaging mistakes that misstate dosage information.
The Medicines and Healthcare products Regulatory Agency (MHRA) announced the recall following an alert from manufacturer CD Pharma Ltd about labelling errors on external packaging.
The affected inhalers contain 250 micrograms of fluticasone propionate and 10 micrograms of formoterol fumarate dihydrate per actuation.
While the packaging fault involves an inaccurate description of the amount of medication delivered with each use, officials confirmed the actual medicine quality remains unchanged. The error affects only printed information on cartons, not the inhaler contents themselves.

The packaging of the inhaler displays an incorrect statement
|GETTY
The packaging displays an incorrect statement claiming each delivered dose contains "approximately 115 micrograms of fluticasone propionate and 4.5 micrograms of formoterol fumarate dihydrate".
However, the accurate delivered dose should state "approximately 230 micrograms of fluticasone propionate and 9 micrograms of formoterol fumarate dihydrate".
This represents a significant discrepancy in the stated medication amounts patients receive with each puff.
The metered dose information remains correctly printed at 250 micrograms and 10 micrograms respectively.
All remaining product details on the packaging, including the medication name, strength and formulation type, contain no errors.
The recall encompasses four specific production batches distributed since late June.
These include batch numbers:
- 270185-24062FA-30424
- 270186-24065FA-30443
- 270774-24082FB-30444
- 269881-24064FA-30425 all containing 120 actuations and expiring between June and July 2026.
Pharmacy staff have received instructions to cease dispensing these batches with immediate effect.
All affected stock must be isolated and returned to suppliers through established return channels.
The MHRA has classified this as a wholesale and pharmacy-level withdrawal, meaning healthcare professionals will manage the recall process directly.
The regulator emphasised that individual patients need not return their inhalers to pharmacies.
The MHRA has reassured patients currently using these inhalers to maintain their prescribed treatment regimen without interruption.
LATEST DEVELOPMENTS
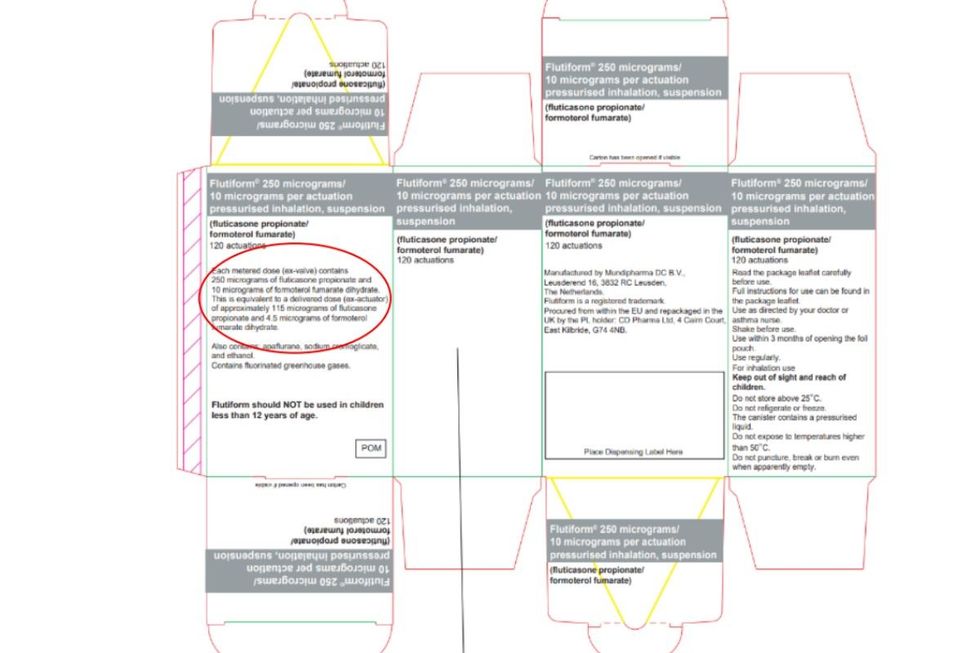
The error appears on the outer carton of the product
|MHRA
"Patients who are taking Flutiform 250 micrograms / 10 micrograms per actuation pressurised inhalation, suspension should continue to take the medication as prescribed by their healthcare professional," the alert stated.
Anyone experiencing unwanted effects or requiring clarification about their medication should contact their doctor or pharmacist.
The regulator has requested that any suspected adverse reactions be documented through the MHRA Yellow Card reporting system.
Officials stressed that the labelling error poses no risk to patient safety, as the inhaler contents remain unaffected by the packaging mistake.