Alzheimer's breakthrough as finger-prick blood test could transform diagnosis, scientists say

Individuals can conduct the test at home and post samples to laboratories without refrigeration
Don't Miss
Most Read
A major international clinical trial is currently investigating whether a simple finger-prick blood test could transform the way Alzheimer's disease is diagnosed.
The Bio-Hermes-002 study spans the United Kingdom, the United States and Canada, and has recruited 1,000 participants aged 60 and above.
Researchers are seeking to identify specific biomarkers in the blood that indicate the presence of the degenerative condition.
The trial is being coordinated by LifeArc, a medical research charity, alongside the Global Alzheimer's Platform Foundation, with additional backing from the UK Dementia Research Institute.
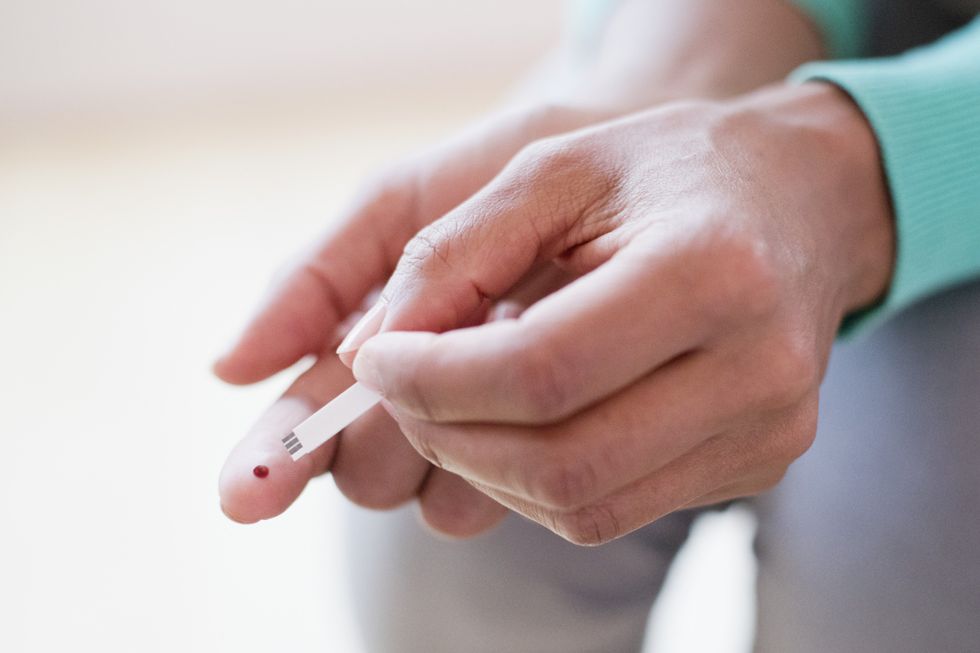
Current assessments for Alzheimer's disease are costly and time-sensitive
|GETTY
Should the research prove successful, it could offer a far less invasive alternative to current diagnostic methods, potentially revolutionising early detection of the most prevalent form of dementia.
The study focuses on detecting three specific proteins in the blood that scientists have linked to Alzheimer's disease.
Dr Giovanna Lalli, director of strategy and operations at LifeArc, said: "By analysing the concentration and the levels of these proteins, it may tell us whether a person is at risk of developing Alzheimer's disease."
Research has demonstrated that harmful proteins known as amyloid and tau can build up in the brain as much as 15 years before any symptoms manifest.
LATEST DEVELOPMENTS
Presently, the gold-standard diagnostic procedures involve either a specialised PET brain scan utilising radioactive tracers or an invasive lumbar puncture to extract cerebrospinal fluid.
These existing methods are costly, time-intensive and uncomfortable, with merely two per cent of Alzheimer's patients currently being offered such assessments.
Dr Michael Sandberg, a general practitioner based in London, joined the trial following his personal experience of watching his mother's gradual deterioration from Alzheimer's.
His motivation stemmed partly from his mother's positive experience participating in a clinical study herself.
Dr Sandberg told the BBC: "I believe knowledge is power and am really excited that you may be able to screen people at risk of dementia without expensive scans or needles."
Upon receiving his results, both the conventional brain scan and the novel finger-prick test came back negative for markers of the disease.
He said: "It's a huge relief, knowing what my mother went through."
Naturally, this represents just a single outcome from the trial, and scientists must evaluate data from all 1,000 participants before drawing conclusions about the test's effectiveness.
Prof Fiona Carragher, chief policy and research officer at the Alzheimer's Society, said, with tests not currently widely available across the UK, "getting an accurate diagnosis takes far too long".
She added: "With new treatments on the horizon, early and accurate diagnosis must be a priority for the NHS. That's why we're funding work to bring blood tests for dementia to the NHS, so everyone who would benefit from a diagnosis can get one quickly and accurately."
Dr Emer MacSweeney, a neuro-radiologist at ReCognition Health who is recruiting British volunteers, highlighted that success would deliver a widely accessible and precise diagnostic tool without complex procedures.
Proteins like Amyloids and Tau build up in the brains of dementia patients years before diagnosis
|GETTY
A significant advantage of the finger-prick approach is that individuals can conduct it at home and post samples to laboratories without refrigeration.
Thus far, 883 volunteers have enrolled, with over 360 having completed all assessments.
The participant group includes those with normal cognition, mild impairment and early-stage Alzheimer's, with at least a quarter drawn from under-represented communities.
Results are anticipated in 2028.
Our Standards: The GB News Editorial Charter