Parents of trans children launch High Court challenge against £10.9million puberty blocker trial

'DISGUSTING LIE!' NHS presses ahead with puberty blocker trial sparking FURY as protests clash |
GB NEWS
The study received ethical approval in November
Don't Miss
Most Read
Trending on GB News
A High Court legal challenge has been launched to stop a £10.9 million puberty blocker trial involving children as young as ten, weeks before recruitment is due to begin.
The case has been brought by parents of children and young adults who identify as transgender, a psychotherapist and 28-year-old de-transitioner Kiera Bell.
They are seeking to block a government-backed trial led by King’s College London, in partnership with the South London and Maudsley NHS Foundation Trust, one of the UK’s largest mental health providers.
The study received ethical approval in November and is due to begin enrolling participants from April.
TRENDING
Stories
Videos
Your Say
The claimants are suing the Health Research Authority, which oversees research ethics in England, and Wes Streeting, Secretary of State for Health and Social Care, who has responsibility for medicines regulation. They argue that approval for the trial was granted unlawfully and should be overturned.
At the centre of the challenge are questions about how ethical approval was granted by a Research Ethics Committee operating under the Health Research Authority.
The claimants argue the committee was not provided with full information about the potential risks of puberty blockers, particularly evidence relating to effects on the brain - “cognitive effects”.
According to the legal claim, the committee was told that “there was no data at present on the cognitive impacts of puberty blockers”.
 The trial could enrol 226 young people between the ages of 10 and nearly 16 who are questioning their gender identity | GETTY
The trial could enrol 226 young people between the ages of 10 and nearly 16 who are questioning their gender identity | GETTYThe claimants say this did not reflect findings from lab studies which reported changes in memory, anxiety, depressive behaviour and the brains of animals exposed to the drugs.
They argue that international research standards require such animal data to be considered before trials involving children are approved.
The claimants also say the committee failed to ensure the trial would offer a clear benefit to participating children.
In documents submitted as part of the approval process, the trial organisers - King’s College London and South London and Maudsley NHS Foundation Trust - acknowledge uncertainty, stating: “We do not know whether the trial may help” participants.
LATEST DEVELOPMENTS
 PICTURED: Pro-trans protests demonstrate against a 2024 ban on puberty blockers | GETTY
PICTURED: Pro-trans protests demonstrate against a 2024 ban on puberty blockers | GETTYPuberty blockers - which suppress the body's production of sex hormones to stop the physical changes of puberty - were restricted for routine use outside research settings in December 2024 by Health Secretary Wes Streeting.
At the time, he said there was insufficient evidence to establish whether the drugs were “safe or beneficial” for children experiencing gender distress.
Supporters of the trial argue that this uncertainty makes clinical research necessary to answer outstanding questions about outcomes and safety.
Opponents argue that risks are already understood and that there are no scientifically proven benefits, making the trial unethical.
Puberty blockers are also known to affect the development of bone density, and campaigners cite data suggesting that around 98 per cent of children who begin blockers proceed to cross-sex hormones which are linked to infertility and sexual dysfunction. They say this “pathway” can also lead children to later consent to more invasive medical treatment with lifelong consequences such as surgery to remove breasts or penises.
The claimants also challenge the design of the trial. They argue it lacks a clear outcome and is unlikely to generate meaningful or reliable data partly because the children receiving the puberty blockers are too young to understand the full implications.
They argue it is inappropriate to conduct such a trial on a group they describe as particularly vulnerable due to their age and the higher prevalence of autism and ADHD among children referred to gender services.
The case is being brought on an “urgent basis by Bayswater Support Group, a parents’ organisation representing around 800 families of children and young adults who identify as transgender or non-binary, psychologist James Esses and de-transitioner Keira Bell.
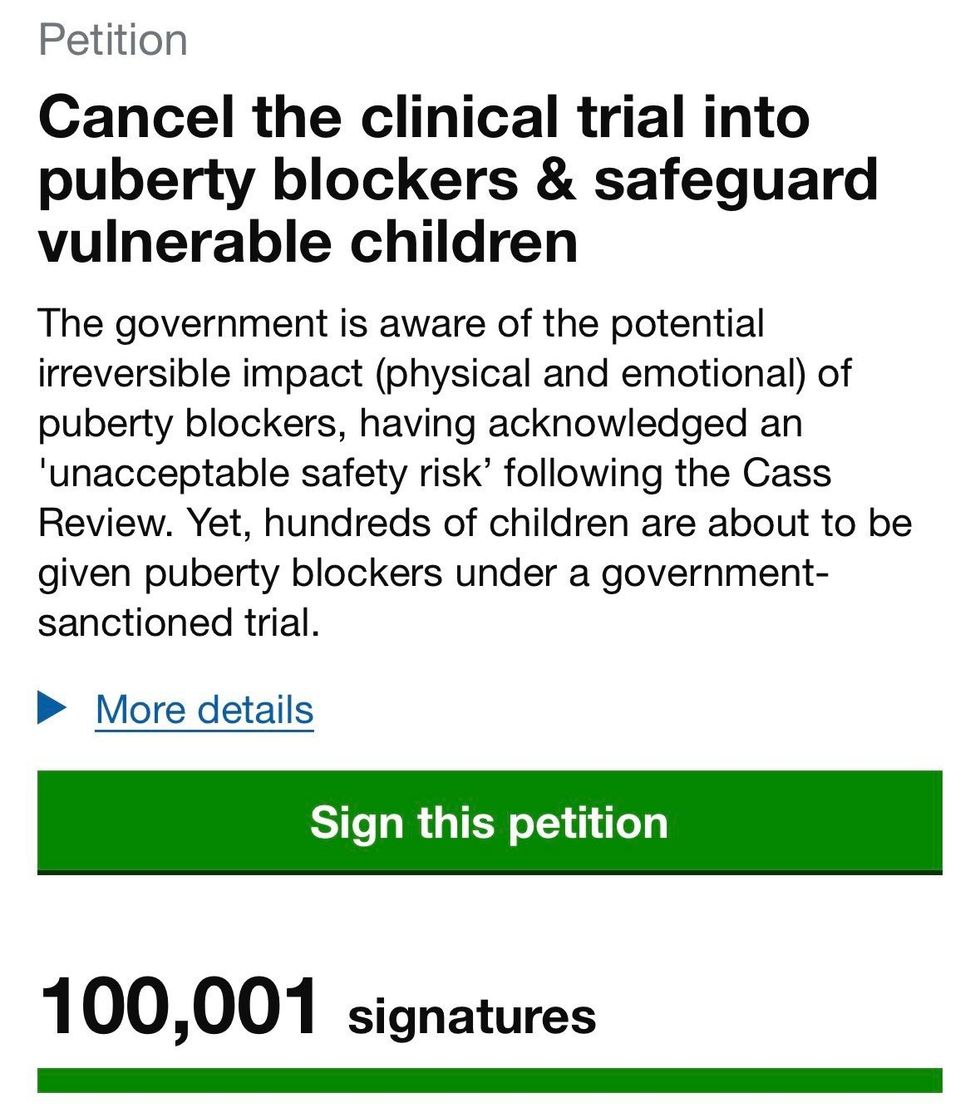 A UK Parliament petition signed by over 100,000 people has called on ministers to cancel the trial | X
A UK Parliament petition signed by over 100,000 people has called on ministers to cancel the trial | XIf necessary, the claimants say they will seek interim order to prevent children being recruited while the court considers the claim.
Other clinicians and members of the public have also expressed concern. A parliamentary petition calling for the trial to be cancelled has exceeded 140,000 signatures, passing the threshold required to trigger a parliamentary debate.
MPs and peers are due to discuss the issue at Westminster on 11 February, where James Esses and Keira Bell are expected to address parliamentarians alongside clinicians and campaigners.
Psychologist James Esses said: “Even in the face of strong evidence of harm, consistent concerns from clinicians, and hundreds of thousands of members of the public petitioning them to stop, it is business as usual,” he said.
He added: “The recruitment of children is due to commence imminently, yet the conspiracy of silence continues. If they won’t safeguard children of their own accord, we will compel them to do so.”
A spokesperson for Bayswater Support Group said the organisation continued to have “serious and unresolved legal, medical and safeguarding concerns” about the trial.
Stephanie Davies-Arai, director of Transgender Trend, a campaign group critical of medical interventions for children with gender distress, said the trial required close scrutiny.
“The risks are serious and lifelong compared to the supposed benefits which are vague, short-term and uncertain,” she said.
A King's College London spokesperson said: "We strongly refute the claim that this carefully designed study is scientifically unsound or that it bypassed the ethics process and we can confirm that the study has completed all the necessary ethics and approvals processes. As part of this process the research team submitted the relevant information to the Research Ethics Committee and has been reviewed by independent scientists.
"Randomised controlled clinical trials are the most robust method to determine whether a medicine or intervention is effective. They are commonly used to understand the benefits and risks of a medicine including for participants under the age of 18.
"Use of puberty suppression to treat young people with gender incongruence has not previously been subject to rigorous evaluation of benefits and risks, despite being prescribed widely. Existing evidence about the impact of puberty suppression to treat young people with gender incongruence is inconsistent.
"PATHWAYS is the first randomised controlled trial to be conducted to understand the impact of puberty suppression on young people with gender incongruence and address this evidence gap. Clinical care should always be underpinned by robust evidence, and this study will help provide a better understanding of how to support young people with gender incongruence."
More From GB News