NHS-backed puberty blocker trial faces High Court challenge amid fears ‘unethical’ study will put hundreds of children at risk
A pre-action legal letter demanding the immediate suspension of the trial has been issued
Don't Miss
Most Read
Trending on GB News
A High Court challenge has been launched to stop a Government-approved clinical trial that plans to give puberty blockers to children as young as 10, with campaigners claiming the study is unethical and will put children at risk of harm.
Three claimants—the Bayswater Support Group, psychotherapist James Esses, and detransitioner Keira Bell—have issued a pre-action legal letter demanding the research authorities suspend the trial immediately. They argue the PATHWAY trial will expose vulnerable children to medical risks without clear evidence of benefit.
The legal action is directed at the Government’s Medicines and Healthcare products Regulatory Agency (MHRA), which licensed the trial, and its Health Research Authority (HRA), which approved its ethics application.
Health Secretary Wes Streeting is named as an Interested Party, along with NHS England, King’s College London, and the South London and Maudsley NHS Foundation Trust, who are jointly responsible for the £10.69m study.
TRENDING
Stories
Videos
Your Say
The PATHWAY trial is set to involve around 226 children aged under 15 years and 11 months. Participants will be randomly assigned either to receive puberty blockers—used to delay or prevent puberty—or to receive them after a one-year delay.
The trial comes after Health Secretary Wes Streeting banned the use of puberty blockers in children in 2024 following a major review.
Before this, the drugs were used to treat some young people with gender incongruence—when someone's gender identity doesn't match the sex they were registered at birth—or those with gender dysphoria, when it causes significant distress.
There is no placebo (dummy drug) arm in the trial. Instead, scientists will assess the outcomes using a self-reported wellbeing questionnaire (KIDSCREEN-10), and the children will also receive bone density scans and assessments of their brain development over a two-year period. Psychological support will run alongside medical treatment. Claimants say the evidence base is weak and that it will be difficult for researchers to isolate the effects of the drugs alone because of the psychological support offered. In their pre-action letter, the claimants also argue puberty blockers are linked with long-term changes to bone density, sexual development, fertility, and potential effects on brain development, and that the trial’s design cannot resolve these safety questions over a long period.
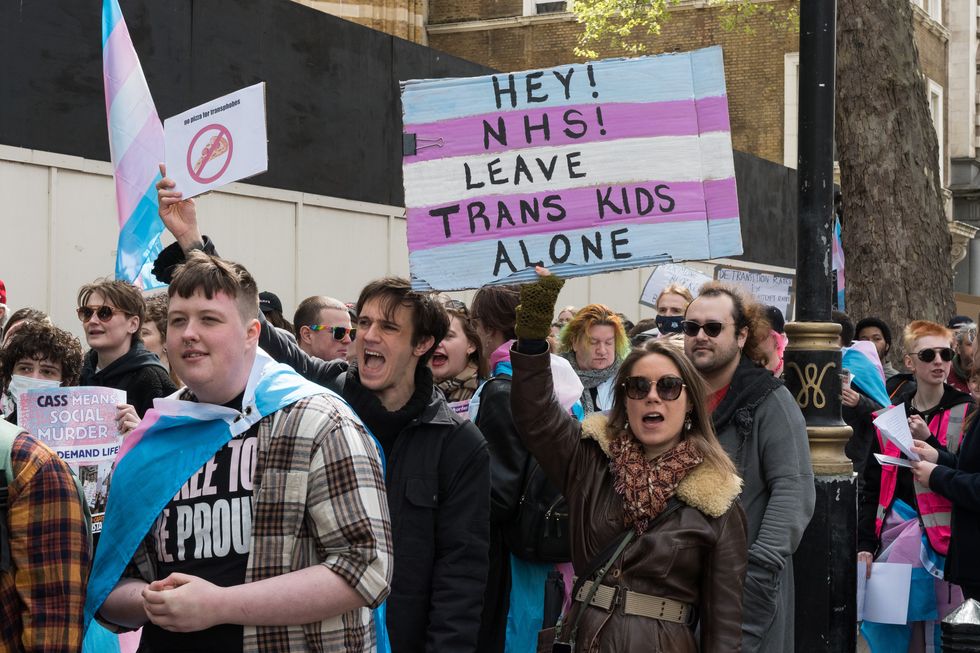 PICTURED: Pro-trans protests demonstrate against a 2024 ban on puberty blockers | GETTY
PICTURED: Pro-trans protests demonstrate against a 2024 ban on puberty blockers | GETTYTheir solicitor, Paul Conrathe, said: “Counselling has been shown to be highly effective for gender dysphoria, and studies demonstrate that over 80 per cent of children who struggle with gender identity will naturally grow out of it. Against this background, this trial of banned drugs on children, many of whom are vulnerable and unable to consent to the treatment, is profoundly concerning. Crucially, the trial has no way of knowing if the treatment will benefit an individual child. It can be certain that it exposes that child to significant risks—infertility, poor bone density, to name but a few.”
He added: “There are grave legal concerns about conducting a trial on children using a treatment that may lead to irreversible lifelong harm, and which research has already shown provides limited, if any, benefit. The failure to conduct animal trials before experimenting on children exposes them to an unacceptable risk of harm.”
A spokesperson for the Bayswater Support Group warned the trial could repeat earlier failures. “The puberty blocker trial will subject a new cohort of children to an experimental intervention. This is despite repeated previous failures to demonstrate benefits and mounting evidence that this is just the first step towards lifelong medicalisation. This comes with a high risk of infertility, sexual dysfunction, and long-term health risks.”
They added: “The trial period is too short to offer meaningful information, and proceeding in this way without tracking outcomes from the previous puberty blocker experiment is indefensible.… Families will continue to self-exclude from NHS paediatric services until the support on offer for children is safe and effective.”
LATEST DEVELOPMENTS
 Protest against ban on puberty blockers in London earlier this year | GETTY
Protest against ban on puberty blockers in London earlier this year | GETTYClaimant Keira Bell, 28, who was given puberty blockers and testosterone as a teenager before detransitioning, said:
“I think it’s disgusting that we're again putting children on these drugs. This is all in the name of a new 'study,' whereas in fact The Tavistock conducted its own study years ago. The Secretary of State then banned these drugs as unsafe. Have we forgotten about the children that have already suffered from puberty blockers?”
Psychotherapist James Esses said: “We already know the irreversible damage, both physical and emotional, caused by puberty blockers. That is precisely why they were banned in the first place. To put more children on a path towards such harm, like lambs to the slaughter, would be the antithesis of child safeguarding. The rest of the world is looking on. Let us not be written into the history books as the country that knowingly destroyed the lives of vulnerable children.”
Stephanie Davies-Arai, of the campaign group Transgender Trend, warned: “The most worrying problem which surfaced in the 2011 trial was the fact that 98 per cent of these children progressed to cross-sex hormones.… Are we going to start this merry-go-round again?”
She added: “Once on cross-sex hormones, these children face a whole lifetime as medical patients.… Blocking the critical growth stage of puberty is medical harm in itself. We don’t need a trial to tell us that.”
The legal challenge comes after a series of official reviews questioning the use of puberty blockers for children. The Cass Review found weak evidence of benefit and raised concerns about potential impacts on bone density, brain development, and psychosexual outcomes.
In December 2024, Health Secretary Wes Streeting brought in a UK-wide permanent ban on the drugs being prescribed privately or by the NHS to children and young people questioning their gender identity.
Mr Streeting told Parliament: “It is a scandal that medicine was given to vulnerable young children without proof that it was safe or effective.… I am treading cautiously in this area because the safety of children must come first.”

Health Secretary Wes Streeting
| POOLThe Commission on Human Medicines later said there was an “unacceptable safety risk” in prescribing puberty blockers to minors outside controlled research settings.
The claimants are asking the MHRA and HRA to suspend the PATHWAY trial or terminate their approval of it.
They want details of when children are due to be recruited, warning that if enrolment is imminent, they may seek an injunction to prevent the study from starting before the High Court rules.
They say unless they have a full response from the regulators by 19 December, they will launch a legal battle.
Prof Emily Simonoff, study leader and professor of child and adolescent psychiatry at King’s College London, said in a previous interview that young people and their parents attending services for gender distress “tell us that they don't know what to do—they look at the information that's out there and they don't know what's best for them.”
She said the study was not expecting a “one size fits all finding.” “We are looking very much at the balance between, possibly, benefits for mental health and quality of life, and any possible risks or harms.”
She added this would include monitoring people's physical health and would also be the first-ever study looking at the impact on brain development.
More From GB News