Doctors urge ministers to halt ‘unethical’ puberty blocker ‘trial’ on children

Clare Muldoon calls for an outright ban on the upcoming puberty blocker trial |
GB NEWS
The trial will be conducted on 226 children, split across two groups
Don't Miss
Most Read
Trending on GB News
Senior doctors, scientists, psychologists and medical ethics experts are urging ministers to halt a controversial NHS puberty blocker trial, warning it risks harming vulnerable children as young as ten with no proven benefit.
At a special parliamentary briefing tomorrow (Thursday, January 15), critics of the drugs will tell MPs the King’s College London PATHWAYS trial should be stopped before recruitment expands, arguing it is unethical, unnecessary and potentially damaging for children who are already distressed and too young to realise the implications of the therapy.
The intervention comes amid mounting political pressure and public concern, including a UK Parliament petition signed by over 100,000 people calling on ministers to cancel the trial and prioritise child safeguarding.
Author JK Rowling revealed she has signed the petition, posting on social media platform X: "I’ve signed. This is an unethical experiment on children who can’t give meaningful consent."
TRENDING
Stories
Videos
Your Say
The petition was also flagged by entrepreneur Elon Musk who posted on his platform X: "Puberty blockers are a crime against children. They should be banned."
It also follows a letter signed by 350 clinicians urging the Government to pause or cancel the trial, warning foreseeable risks outweigh any potential benefits.
One speaker at the briefing is Oxford University academic Michael Biggs, will highlight the damaging effects of puberty blockers.
Prof Biggs is expected to tell MPs there is no robust evidence that puberty blockers improve children’s mental health, and that UK and US studies attempting to replicate a small-scale early positive finding failed to show benefit.

Ministers have been told to stop the trial over health and safeguarding fears
| GETTY“The first Dutch study found psychological improvempeent,” he has said previously, “but attempts to replicate it - including by the (former UK gender service) Tavistock itself - did not find those benefits.”
He will also warn of physical harm, including reduced bone density - much of adult bone strength is built during puberty.
Prof Biggs has shown that after two years of puberty suppression, “up to a third” of youngsters had abnormally low bone density, placing them in the lowest 2.3 per cent for their age and sex, with some recording extremely low values.
He also highlighted the case of a Swedish adolescent who developed the brittle bone condition osteoporosis after being placed on puberty blockers and had to stop the drugs as a result.
Prof Biggs told GB News ministers should prioritise following up the nine thousand young people previously treated at the former Tavistock gender clinic, rather than recruiting new children into an experimental trial.
Prof Biggs says the youngsters in the trial, which will involve 262 pre-pubescent children as young as ten, are not old enough to consent properly. He said: “How can a child who is not sexually active agree to not having a proper sex life as an adult? There is no ethical justification for this trial given what we know.”
Also speaking will be Louise Irvine, a retired GP and co-chair of the Clinical Advisory Network on Sex and Gender.
Dr Irvine will argue the trial fails basic ethical tests applied to medical research involving children.
She told GB News: “This is introducing harm into the normal physical development of healthy children.
“Puberty is a crucial stage of human development, and deliberately stopping it carries risks to bone health, brain development, sexual development and fertility.”
She has also questioned the trial’s design, warning that short-term wellbeing scores cannot justify long-term developmental risk.
“This trial is so short it will not show anything useful,” she said. “We already know puberty blockers stop the normal development of bone density, increasing the risk of osteoporosis and fractures in adult life. We don’t know how much recovery is possible later - and you cannot ethically trade that risk for a subjective wellbeing score at two years.”
LATEST DEVELOPMENTS:
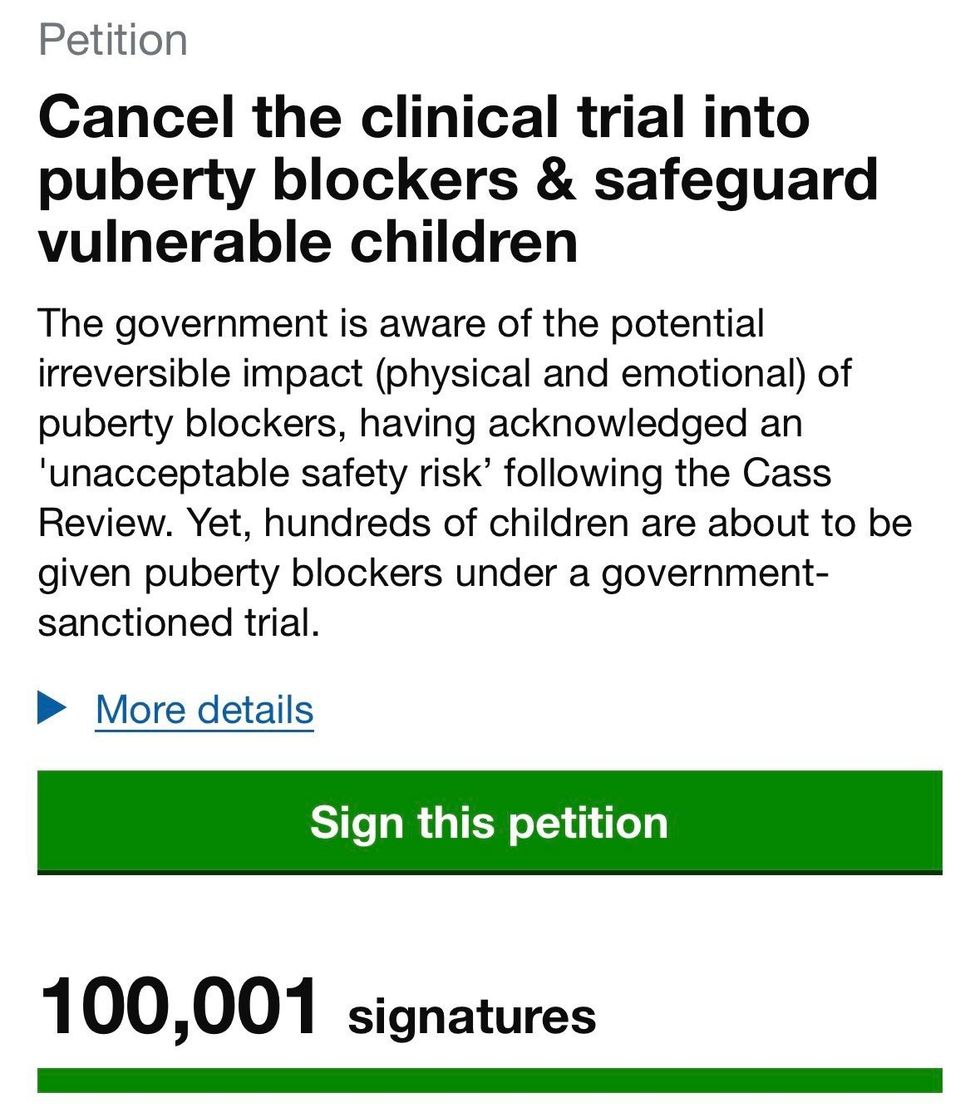
A UK Parliament petition signed by over 100,000 people has called on ministers to cancel the trial
|X
Clinical researcher Sinead Helyar, who has worked on trials involving children and vulnerable patients for over 15 years, will say the study does not meet standard safety criteria.
She said: “In clinical trials, potential benefits must outweigh risk. These are physically healthy, potentially fertile children. Introducing developmental harm cannot be justified.”
Dr Helyar warned pre-clinical animal studies were not adequately considered.
“We are concerned about bone development, brain development and sexual development,” she said. “Animal studies show puberty blockers permanently impair cognitive function. That alone should have triggered extreme caution.”
Campaigners also point to research showing most children overcome gender distress during puberty and point to the experience of Keira Bell, whose case became a turning point in the UK debate.
Ms Bell, 28, was prescribed puberty blockers at 16 at the NHS Tavistock gender clinic, before progressing to cross-sex hormones and a double mastectomy. In her twenties, she detransitioned, saying the medical pathway failed to address the underlying causes of her distress.
She took a case to the High Court saying she had not been properly challenged or offered psychological alternatives and that she regretted the treatment, which has left her with permanent facial hair, a deepened voice and sexual dysfunction.
Her case helped expose serious gaps in evidence, follow-up and safeguarding, triggering wider scrutiny of the Tavistock service and paving the way for the independent Cass Review.
Psychologist James Esses, who has worked with children distressed about their bodies and identity is one prominent critic of the trial.
He said: “This is state-sanctioned child abuse. We already have a number of studies that demonstrate damage caused by puberty blockers and the fact there is no good evidence of benefit means this is being driven by ideology rather than science.”
He added: “These children cannot consent. Many are so desperate they will say whatever it takes to get the drugs. That does not make it ethical.”
The trial will randomly allocate 226 children into two groups - one receiving puberty blockers immediately and the other after a 12-month delay. Participants will be followed for up to two years, a timeframe critics say is too short to capture long-term physical, neurological or reproductive outcomes.
Puberty blockers suppress the body’s release of sex hormones, arresting pubertal development while they are taken.
Critics argue that while presented as temporary, most children who start them proceed to cross-sex hormones, which induce physical changes associated with the opposite sex including permanent changes to reproductive organs and infertility.
The trial was recommended by the Cass Review published in April 2024, which concluded the evidence base for puberty blockers was weak and uncertain. It was one of 32 recommendations, alongside psychological support, long-term follow-up studies and services for detransitioners.
Following the review, Health Secretary Wes Streeting moved in December 2024 to halt the routine prescribing of puberty blockers to under-18s, restricting their use to approved clinical trials only, citing concerns about safety, consent and lack of evidence.
Supporters say the trial is necessary to resolve uncertainty.
An NHS spokesman said: “These important research studies will help us to better understand how to support young people with gender incongruence within the NHS as we continue to implement the recommendations from the Cass Review.
“Building on advice from Dr Cass, the clinical trial will gather vital evidence on the potential use of puberty suppressing hormones for children and young people, alongside comprehensive assessment and psychosocial support, so we can continue to deliver care that is safe, effective and evidence-based, while also helping young people and their families make more informed decisions about their care.
“The PATHWAYS research programme has been designed to the highest scientific and ethical standards, with the wellbeing of young people at its heart.”
Our Standards: The GB News Editorial Charter
More From GB News