Huntington's disease: Scientists celebrate 'breathtaking' treatment that slows progression by 75%

Patients could potentially enjoy additional decades of meaningful life with the treatment
Don't Miss
Most Read
Latest
Medical researchers have announced a revolutionary breakthrough in treating Huntington's disease, achieving a remarkable 75 per cent reduction in the rate at which the condition advances. This marks the first time doctors have successfully slowed this fatal inherited neurological disorder.
The innovative gene therapy means deterioration typically seen over 12 months now takes four years to occur, potentially providing patients with additional decades of meaningful life.
Professor Sarah Tabrizi from University College London's Huntington's Disease Centre called the findings "spectacular", stating: "We never in our wildest dreams would have expected a 75 per cent slowing of clinical progression."
The condition, which affects approximately 75,000 individuals across Britain, America and Europe, typically manifests during patients' 30s or 40s and proves fatal within 20 years.
TRENDING
Stories
Videos
Your Say
Huntington's Disease affects approximately 75,000 individuals across Britain
|GETTY
The groundbreaking treatment requires an intricate neurosurgical procedure lasting between 12 and 18 hours. Surgeons use real-time MRI scanning to guide a microcatheter into specific brain areas, delivering specially engineered genetic material directly to neurons.
This genetic intervention transforms brain cells into permanent production sites for therapeutic molecules. The modified cells continuously manufacture substances that neutralise the harmful proteins responsible for the disease's devastating effects.
Professor Ed Wild, consultant neurologist at the National Hospital for Neurology and Neurosurgery at UCLH, described the results as "breathtaking".
He said: "There was every chance that we would never see a result like this, so to be living in a world where we know this is not only possible, but the actual magnitude of the effect is breathtaking; it's very difficult to fully encapsulate the emotion."
The therapy offers renewed optimism for families devastated by this hereditary condition. Jack May-Davis, aged 30, carries the genetic mutation that claimed his father Fred's life at 54 and previously affected his grandmother Joyce.
Fred's symptoms emerged in his late 30s, progressively affecting his movement and behaviour until he required round-the-clock palliative care. Jack described witnessing his father's decline as "really awful and horrible".
Despite knowing he faced the same destiny, Jack participated in research at UCL. Now working as a barrister's clerk and recently engaged to Chloe, he described the breakthrough as "absolutely incredible".
"It does allow me to think my life could be that much longer," he said, adding that his future "seems a little bit brighter" following today's announcement.
The treatment employs a specially modified virus as a delivery vehicle for therapeutic DNA sequences. This harmless virus carries genetic instructions deep into two critical brain areas - the caudate nucleus and putamen.
Once inside neurons, the virus delivers its genetic cargo, which becomes permanently integrated. The cells then begin producing small RNA molecules designed to intercept and neutralise the faulty instructions that create toxic huntingtin protein.
This molecular intervention prevents the manufacture of the harmful protein that destroys brain tissue. The therapy essentially reprogrammes neurons to protect themselves from the disease's destructive effects.
Evidence from spinal fluid analysis confirms the treatment preserves brain cells. Markers indicating neuronal death, which typically increase by a third as the disease advances, actually decreased following the intervention.
The 29-patient trial data, released by pharmaceutical company uniQure, demonstrates sustained benefits three years post-treatment. Some participants experienced temporary inflammation, causing headaches and confusion, which either resolved naturally or responded to steroid therapy.
LATEST DEVELOPMENTS
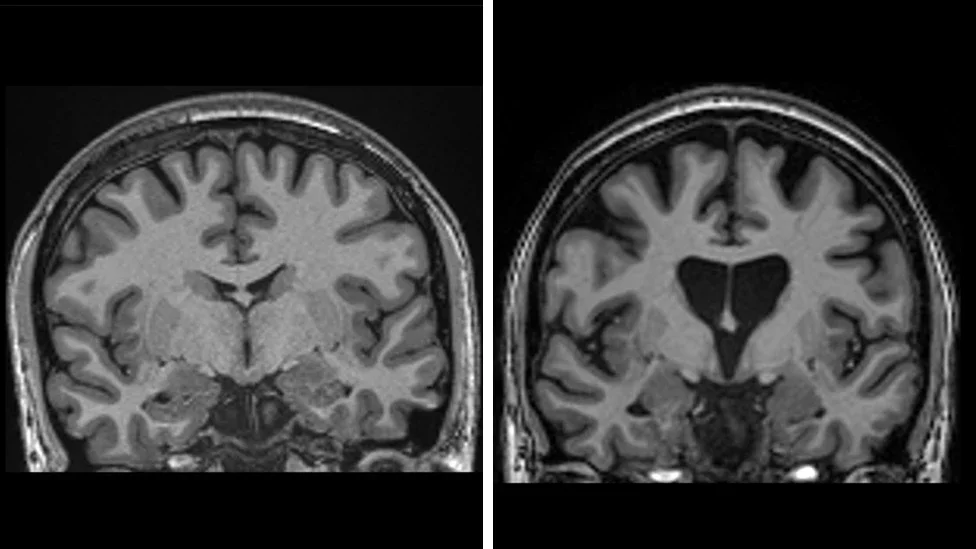
The brain scan on the left shows a healthy brain, while the one on the right shows a loss of brain matter due to the death of neurons
|UCLH
One previously medically retired patient has returned to employment, while others maintain mobility despite predictions that they would require wheelchairs. Professor Wild anticipates the therapy "should last for life" as brain cells don't regenerate like other body tissues.
UniQure plans to seek American regulatory approval in early 2026, aiming for market launch later that year. British and European regulatory discussions will commence next year.
Dr Walid Abi-Saab, UniQure's chief medical officer, said the treatment had "the potential to fundamentally transform" Huntington's disease.
However, the procedure's complexity and anticipated high costs will limit accessibility, with Professor Wild acknowledging "it will be expensive for sure".
Our Standards: The GB News Editorial Charter